
“The Building Blocks of the Universe.” When you put it that way, atoms sound less like a topic specifically for a chemistry class and more like something astronomers might discuss.
They really are. I’ve got a fantastic reason to include atoms under astronomy, and its name is stellar spectra.
We’ve encountered stellar spectra before in these astronomy posts. When I wrote about the spectrograph, an instrument astronomers use to study data, I talked about spectral lines. I also promised we’d come back to elaborate on that later.
We’re not actually going to talk about the spectrograph in this post. I’m saving that for another time. For now, I’m going to cover atoms in a little more detail.
That way, we’ll have a better understanding of how they interact with light later on—and that will help us understand the spectrograph.
You have probably seen a diagram like this before.
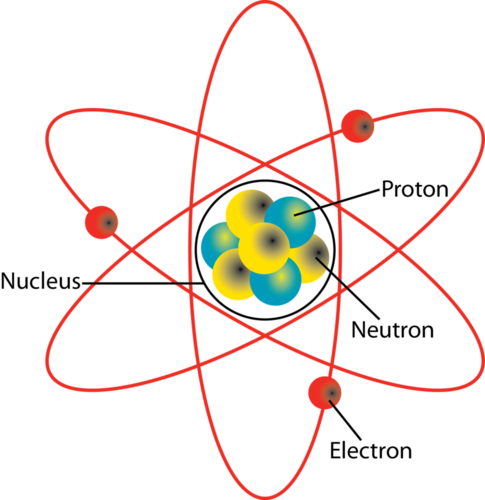
Basically every science website there ever was has used this diagram as a logo, as part of a product, or in some other way. It’s all over the internet—just search up “atom diagram” and you’ll see this. But what is it? And how accurate is it?
As it turns out…it’s not actually very accurate at all.
That’s because it’s a model of an atom.
Scientific models can be inaccurate and still useful to help us understand something. Just look at the celestial sphere—the universe doesn’t really form a finite sphere around the Earth. But it looks like it does to us humans, and it’s useful to help point a telescope.
Understanding the atom is incredibly important to science. If we can understand the building blocks of the universe, imagine how many of its secrets we can unlock. But we can’t see them. So we use a simple model to help us picture how they work.
What they really look like, though…that’s a different story.
Here’s a traditional Bohr model of an atom. (Who the heck is Bohr, and are there other types of models of the atom? You’ll find out through my chemistry posts…eventually.)
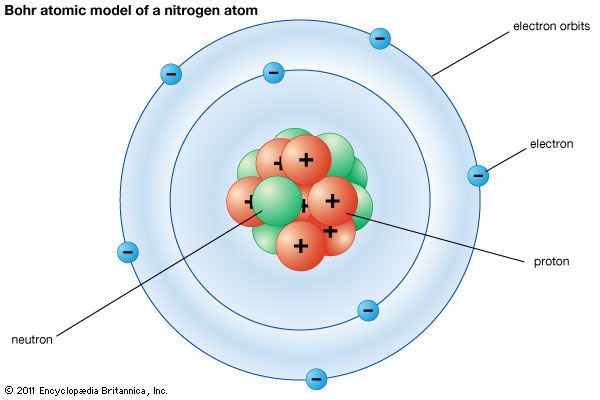
This model exists for two basic purposes. It helps you picture the parts of an atom, and it helps you understand how atoms interact. But it doesn’t explain why they behave that way. There are other models of the atom that do that better.
So what are the parts of an atom?
For this post, we’re only going to deal with the most basic parts. There are much smaller particles that make up these three particles. But the particles we care about are protons, neutrons, and electrons.
You may have heard of these before.
Protons and neutrons are close to the same size and make up what we call the nucleus of an atom. They actually make up most of the mass—the “stuff”—in an atom.
Would it surprise you to hear that an atom is mostly empty space?
That’s right. And to show you just how much empty space, I’m going to make a scale model of an atom.
Let’s imagine that a proton is the size of a single grape seed. (It’s not. It’s about a trillion times smaller.)

Now, let’s imagine a football field. (Yes, I promise I’m going somewhere with this.)

Now, we only care about the part of the football field that’s outlined in white paint. So, only about 100 meters of it. That’s still a whole lot of football field.
Now imagine three and a half more football fields, laid end to end. So now we’re talking 450 meters.
Now stick your grape seed in the center, and draw a circle that’s four and a half football fields across.
…that’s the scale of an atom.
So what’s in all that empty space?
Well, that’s where the electrons are. And electrons are tough things to figure out. They’re so tiny, even compared to atoms themselves, that we don’t even include them when we measure an atom’s mass.
How big are atoms, anyway?
The smallest is about a trillionth of a football field. 400 meters divided by a trillion…that’s 4 x 10-10 m. Which is 0.4 nm, short for nanometers…okay, let’s just say, atoms are tiny. Thousands can fit in a single pinhead.

So…back to electrons. What exactly are they?
They’re a mystery, that’s what they are.
In diagrams, we draw them as little beads orbiting the nucleus. But the fact is, one basic law of quantum mechanics—the study of the atomic world—is that you cannot simultaneously know the exact location and motion of a particle. That includes electrons.
They’re not like cars. We can’t measure their speed from point A to point B. We can’t point to them on a city map. What we can do is describe what their behavior means for atoms…and I’ll talk about that in a couple posts.
Here’s what you can take away from this post. Each atomic particle is known by its electric charge.
Protons are positive. They attract an equal number of electrons, since electrons are negative. That’s how many electrons you’ll find in any one atom.
Neutrons are neutral, meaning they don’t have a charge. They don’t affect how many electrons an atom has, but they sure do add to the mass—they double it from what it would be if there were only protons.
I just described an atom to you. But the universe is made up of atoms. How can we have different materials if everything’s made up of the same thing?
There are different kinds of atoms. Over a hundred different kinds. And I’ll cover that in my next post.
They are back! So happy! Need to read again though…. Did not make it all the way to the end….
LikeLiked by 1 person
Haha. They are indeed back, and there are tons more coming. Let me know what you think when you get to the end!
LikeLiked by 1 person
Will do!
LikeLiked by 1 person
Took me three sittings, but I made it through. Thanks for the walk-through. Got it. Ions. Isotopes. Quantum mechanics. Protons, Electrons, Neutrons. It’s been a long time since high school science. Cheers —
LikeLiked by 2 people
I’m glad I was able to inform. How’s the general readability? Do I need to explain better in future chronicle posts?
LikeLike
You explain very well, and your graphics are great. Many (most?) blog readers won’t take the time to get through a very long post. Who is your intended audience? If it’s non-scientists like me, with limited time, this could have been three posts — proton-electron-neutron; ions-isotopes; binding forces. Keep using lots of everyday metaphors, too. Very helpful.
LikeLiked by 1 person
The audience is just about anyone who wants to read. I’m trying to make science easy-access. People don’t have to pay for a class or rummage through their attic for their old 11th grade notebook—they can just come down to my blog and read a few astronomy posts for free.
LikeLiked by 1 person
Wow thanks for the reblog!
LikeLike