
Does this image look familiar?
It should—these are soap bubbles.
Okay, now you’re probably going to ask me how soap bubbles have anything to do with the battery of the sun.
Well…you might be surprised to know that soap bubbles actually work as models of stars.
How?
Have you ever wondered how a soap bubble is held together?
You might jokingly answer, “Not for long,” and you’d actually have a point. A soap bubble is held together by two opposing forces, and they don’t usually stay balanced very well. When one wins out, the bubble pops.
When we’re talking about bubbles, these two opposing forces are the pressure of the air inside against the surface, and the pressure of the air outside against the bubble. When we’re talking about stars, though, the two opposing forces are a little bit different.
The gases in a star are far more energized than those in a bubble, so the star naturally wants to expand outward. What keeps it intact is its own gravity, holding those gases together.
You can imagine how a star might die—if its own internal pressures win out, it’ll go supernova. But if if gravity wins out, it’ll condense itself into an infinitely dense point of matter known as a black hole.

Yeah, one of these guys.
But that’s enough on how a star can die. For now, let’s focus on how a star lives—the battery that keeps it running for billions and billions of years.
Stars rely on one of four basic forces in nature: the force of gravity, the electromagnetic force, the weak force, and the strong force.
The electromagnetic force is what keeps fires burning. Gravity rules supreme over the universe. The weak force is involved in radioactive decay—which, yes, I’ll talk more about in future posts. But the strong force is the force that governs stars.
So what exactly is the strong force, anyway?
You might wonder, based on its name, if it’s particularly strong. And you’d be on the right track—the strong force and the weak force are the two strongest forces in nature. (Bit weird to name a strong force “weak,” I know. Scientists are funny that way.)
But here’s the catch. These two forces work only over very short distances. And I mean very short distances. Like…the distance across the nucleus of an atom.
That’s a really, really, unbelievably short distance.
Wait a second…what’s an atom again?
Here’s one—a nitrogen atom.
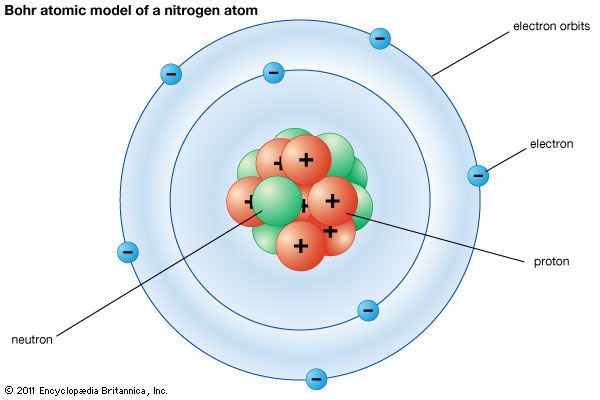
Atoms are the building blocks of the universe. Everything you’ve ever seen in your life is made up of atoms. More complex materials are made up of molecules—which are, in turn, made up of atoms.
A single atom is so tiny, thousands could fit lined up across a pinhead. And an atom itself is mostly empty space. Imagine that—most of the world is made up of empty space.
An atom is made up of three particles: protons, neutrons, and electrons. Electrons are responsible for bonding atoms into molecules and holding the universe together. But when we’re talking about stars, protons and neutrons have a more important job.
Protons and neutrons together make up the nucleus of an atom. It’s that round clump of protons and neutrons in that nitrogen atom above. The number of protons in a nucleus controls what kind of atom it is—and thus, what kinds of materials it can make.
So let’s take a closer look at the nucleus of an atom.

I love it when I can find animations like these, and this is a particularly good one.
The red balls represent protons, and the other ones represent neutrons. Protons have a positive electrostatic charge, so they repel each other the same way as the like ends of two magnets. Neutrons, on the other hand, have no charge.
How the heck do all those protons stay bound together, you might ask?
It’s a good question. The answer is the strong force. Like I said, it’s one of the two strongest forces in nature. It’s strong enough to overcome the repulsion between particles of the same charge.
Explains why this nucleus is jiggling around so much, doesn’t it? Those protons are trying to break free, but the strong force won’t let them.
Notice that atomic nuclei themselves are like our bubble model: a careful balance between two opposing forces. The protons want to repel one another, but the strong force ultimately holds the nucleus together.
There’s a lot of energy involved in this. And there’s two ways to release it: nuclear fusion and nuclear fission.
I know. It’s another case of scientists naming things weirdly. These two methods are completely opposite, but the words look very similar.
Humans have already figured out how to harness nuclear fission. Well…we’re still working on it.

This is what happens when we mess up.
In nuclear fission, scientists take a heavy nucleus—such as the one in the animation above—and split it into two lighter nuclei that are each about half the size of the original. As you can imagine, this releases tremendous amounts of energy.
Stars are very good at using the energy contained within an atom’s nucleus. In fact, they’re so good at it they can’t just power whole planets—they can power whole solar systems. But they don’t use nuclear fission.
They use nuclear fusion. Fusion is a bit different—it involves fusing light nuclei together into heavier nuclei. And humans have figured out how to do this.

In this particular case, we take two isotopes of hydrogen and fuse them.
Because the type of atom only depends on the number of protons, sometimes the same kind of atom can have different numbers of neutrons. That’s the case here—we’re fusing an isotope with one neutron with an isotope that has two neutrons.
Trust me, both deuterium and tritium are isotopes of hydrogen. They’re just not labeled as hydrogen here.
When we crash these two hydrogen atoms together, overcoming the repulsive force between the protons generates energy. So our end products are helium, an extra neutron, and energy.
Stars do this a little differently, but it’s the same basic concept. We call their style the proton-proton chain, and I’ll cover it in my next post.
Excellent article. I marvel at the knowledge, like crowded electrons, that must be spinning in your head.
LikeLiked by 1 person
Haha thanks. Stay tuned for hydrogen fusion! I hope to write that one Saturday. It’s on my editorial calendar.
LikeLike
Where you been? I’ve missed my science.
LikeLiked by 1 person
Been busy. I’m taking a new educational avenue (creative writing now, with an astronomy minor) and it’s a ton of work. I regret that I’ve had to neglect my blog. But now I’ve created an editorial calendar and published it (https://perseshow.wordpress.com/blogging-calendar/) so you can always see what I have planned when. Never fear, the science continues!
LikeLike